The crystallinity of ceramic materials ranges from highly oriented to semi crystalline vitrified and often completely amorphous e g glasses.
What causes the lack of plasticity in crystalline ceramics.
Exceptional plasticity in the bulk single crystalline van der waals semiconductor inse tian ran wei1 2.
Usually plastic deformation of ceramics can be only achieved at high temperature or under superimposed hydrostatic compression since the additional plasticity mechanism is activated or the propagation of cracks that causes premature fracture is impeded.
The lack of plasticity in crystalline ceramics is attributed to their ionic and covalent chemical bonds.
Wang et al 2019.
The lack of intrinsic plasticity and deformability restricts the scope of application for bulk inorganic semiconductors.
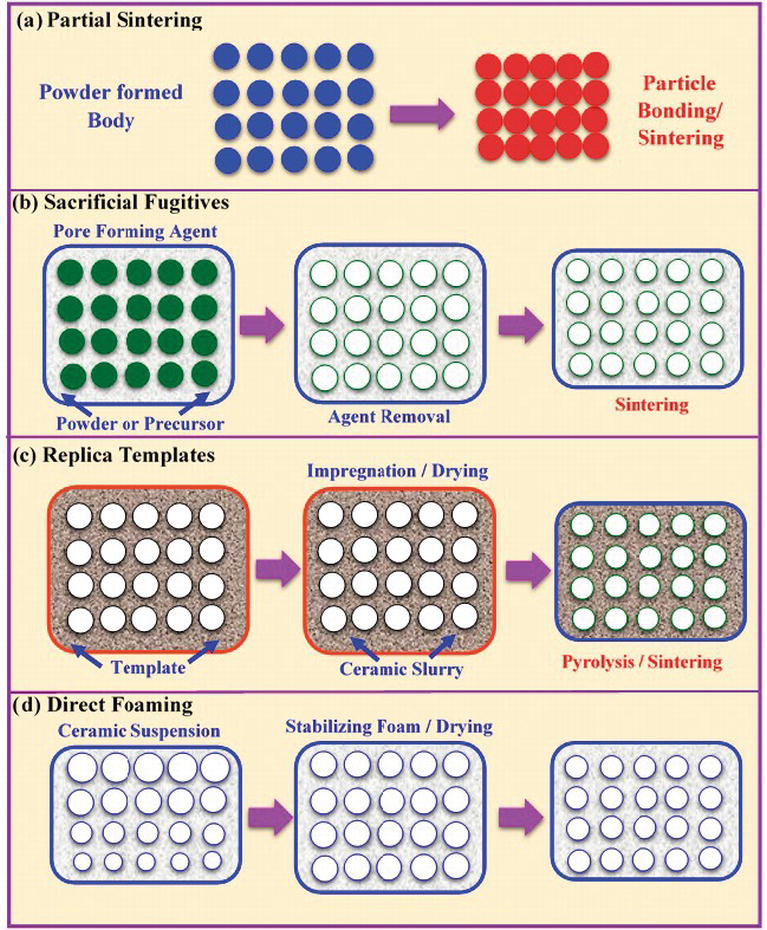
Now look within the category of ceramics.
Biokeram has found an effective way to improve low plasticity ceramics.
Foundations of materials science and engineering 5th edition edit edition.
Lack of intrinsic plasticity and deformability restricts the scope of application for bulk in.
What causes the lack of plasticity in crystalline ceramics.
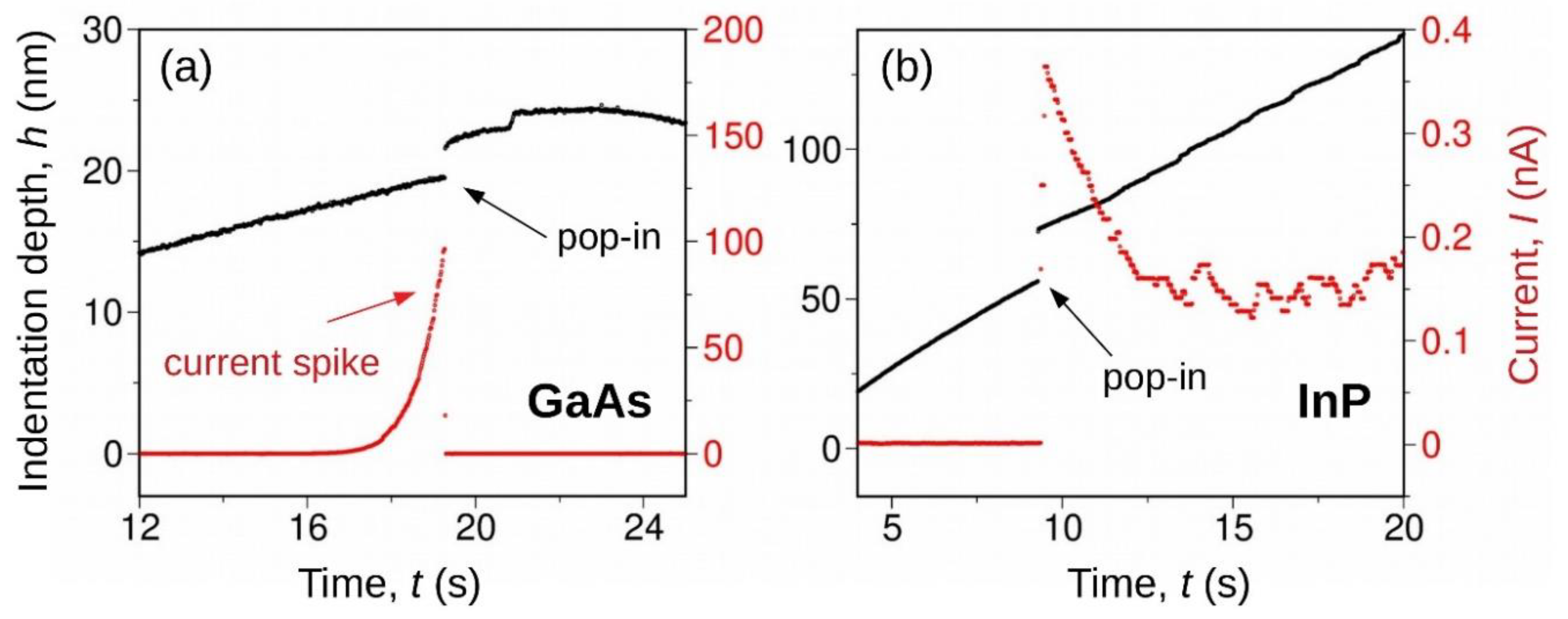
Crystal plasticity is an inherently multi scale process starting at the atomic scale where dislocation cores the regions in the immediate vicinity of dislocation lines control a number of local properties including the selection of glide planes and corresponding dislocation mobility cross slip and nucleation processes 1 5.
What causes the lack of plasticity in crystalline ceramics.
Of high performance ceramics and superfine microstructure shanghai institute of ceramics chinese academy of.
What causes the lack of plasticity in crystalline ceramics.
While doping or alloying may alter the electrical magnetic optical and thermal properties of an inorganic semiconductor by orders of magnitude doping hardly bestows any plasticity and deformability.
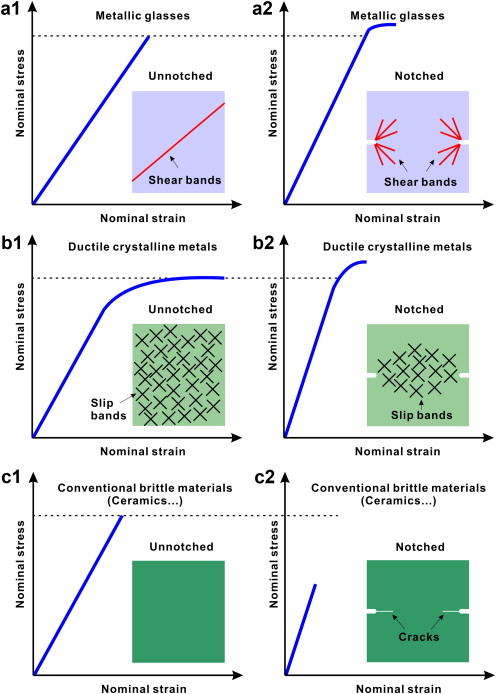
Click but above tg the non crystalline phase becomes ductile.
Common examples are earthenware porcelain and brick.
This problem has been solved.
Problem 86p from chapter 11.
Click 100 crystalline materials are brittle above and below tg.
In covalent crystals and covalently bonded ceramics atoms bond through the exchange of electron charge between pairs of electrons in a specific and directional manner.
A ceramic is any of the various hard brittle heat resistant and corrosion resistant materials made by shaping and then firing a nonmetallic mineral such as clay at a high temperature.
Click totally non crystalline material is brittle below tg and ductile above it.