Fluorine chlorine and all of the noble gases are gases at room temperature and 1 atmosphere pressure.
What gas is monatomic at room temperature and pressure.
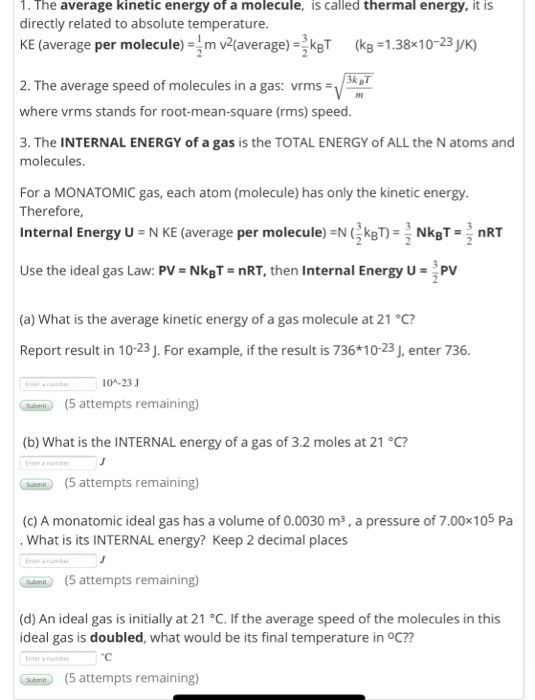
Monatomic gas internal energy.
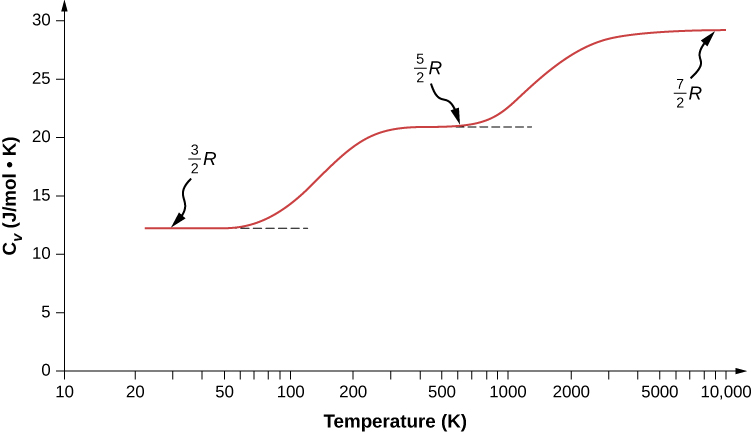
For a monatomic ideal gas such as helium neon or argon the only contribution to the energy comes from translational kinetic energy the average translational kinetic energy of a single atom depends only on the gas temperature and is given by equation.
K avg 3 2 kt.
Easily calculate the pressure volume temperature or quantity in moles of a gas using this combined gas law calculator boyle s law calculator charles s law calculator avogadro s law calculator and gay lussac s law calculator in one supports a variety of input metrics such as celsius fahrenheit kelvin pascals bars atmospheres and volume in both metric and.
The pressure p volume v mole number n and temperature t are related via the equation of state nrt rt p 5 v vmole where vmole is the molar volume defined as the volume occupied by one mole of a substance at a given temperature and pressure and r 8.
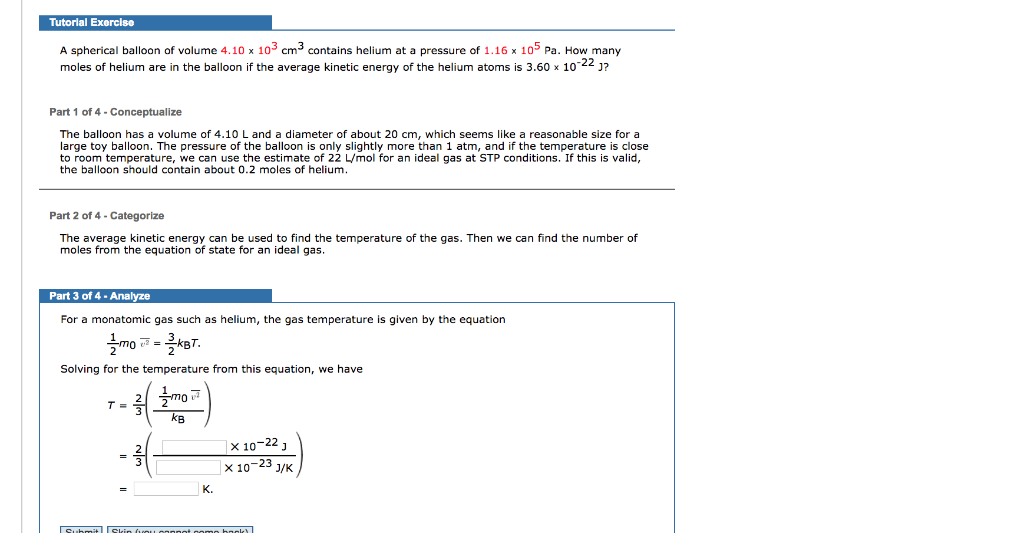
In this problem we will consider a low density monatomic helium gas at room temperature.
The pressure a gas would create if it occupied the total volume available is called the gas s partial pressure.
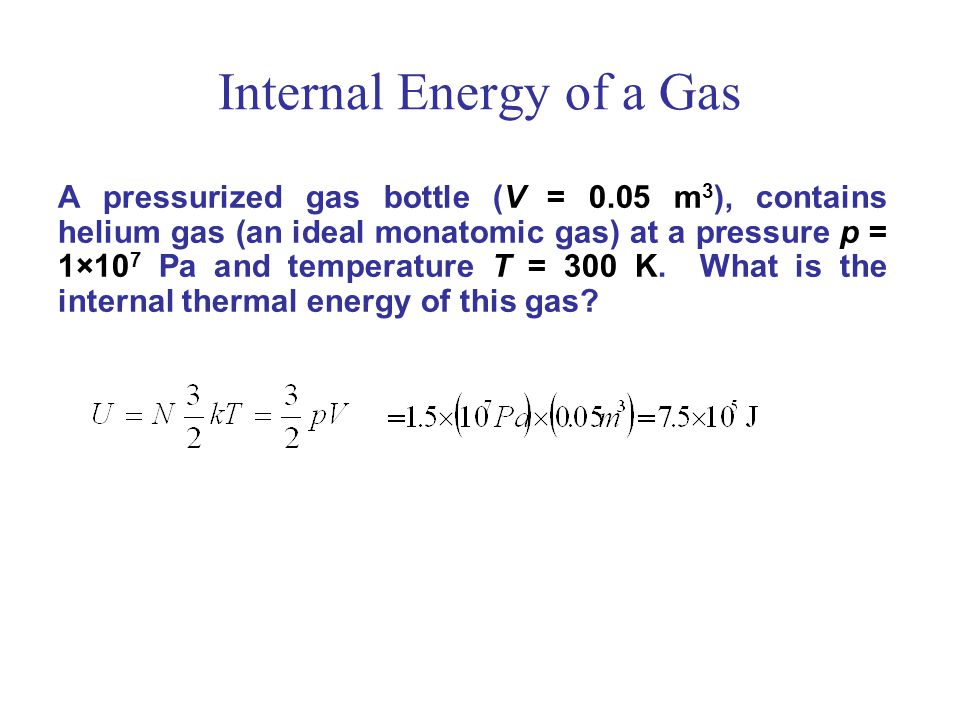
The internal energy of n moles of an ideal monatomic one atom per molecule gas is.
If two or more gases are mixed they will come to thermal equilibrium as a result of collisions between molecules.
Which gas is monatomic at room temperature and pressure nitrogen neon fluorine or chlorine.
Ideal gas law calculator.