The following table summarizes the expected outcome of alkyl halide reactions with nucleophiles.
What are vinylic halides and alkyl.
Formally this is ethylene h 2c ch 2 with one of the hydrogens substituted by a heteroatom.
Other articles where vinylic halide is discussed.
A vinyl halide is clearly a species with a formula h 2c c x h in which a halide is directly bound to an olefinic bond.
In vinylic halides the carbon that bears the halogen is doubly bonded to another carbon.
Vinylic halides may be converted to grignard reagents by reaction with magnesium and these reagents undergo the same types of reaction as those derived from alkyl halides.
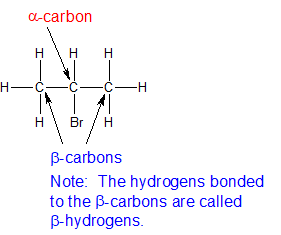
It is assumed that the alkyl halides have one or more beta hydrogens making elimination possible.
The student asked why do vinyl halides not do the s n 2 reaction my answer was that two reasons exist for why the vinyl halide will not react with a nucleophile.
For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides.
Vinyl chloride h 2c chcl is an example.
From the perspective of applications the dominant member of this class of compounds is vinyl chloride which is produced on the scale of millions of.
And that low dielectric solvents e g.
Acetone ethanol tetrahydrofuran ethyl acetate are used.
Vinylic halides resemble alkenes in that they undergo addition to their double bond.
In alkyl halides all four bonds to the carbon that bears the halogen are single bonds.
In organic chemistry a vinyl halide is a compound with the formula ch 2 chx x halide the term vinyl is often used to describe any alkenyl group.
In aryl halides the halogen bearing carbon is part of.
An example is the addition of hydrogen chloride to vinyl chloride to yield 1 1.
They are subdivided into alkyl vinylic aryl and acyl halides.